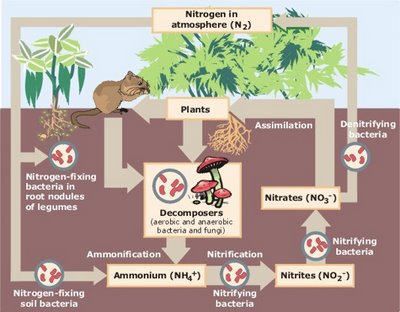
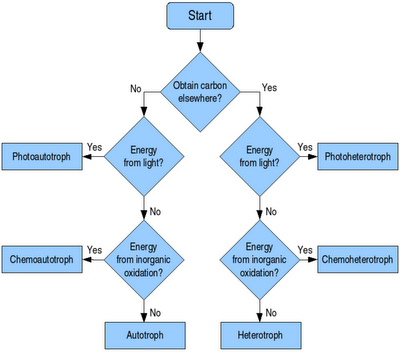
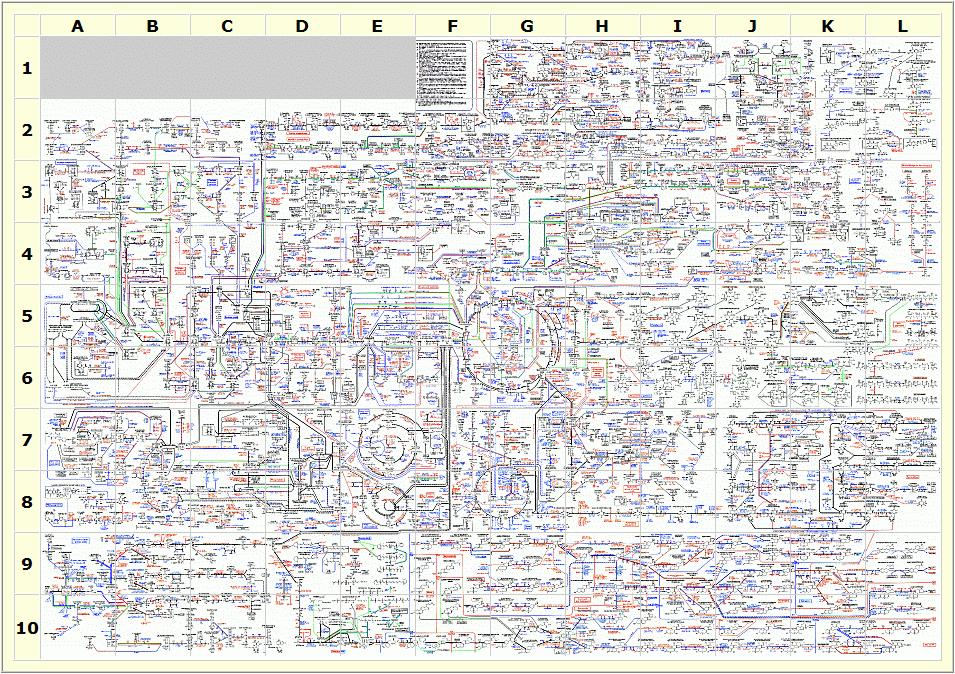
Electron Transport Chain vs Oxidative Phosphorylation
| oxidative phosphorylation | ||
| energy input / usage | utilizes products of the Krebs cycle | driven by the process of proton pumping by complexes I, III, and IV |
| phosphorylation / dephosphorylation | generates ATP from energy stored by the electron transport chain (ETS) | consumes NADPH & FADH2 |
| proton pump | ultimately coupled to phosphorylation | electron transport is driven by the proximity of reduced and oxidized carriers generated by proton pumping |
| electron transport | complexes I, III, and IV pump protons coupled to proton pumping ... you don't get one without the other |
electrons enter the chain only through NADH dehydrogenase and succinate dehydrogenase electron transport is driven by the proximity of reduced and oxidized carriers generated by proton pumping |
| chemiosmotic gradient | maintained in presence of substrate, and in absence of mitochondrial poisoning by chemicals such as cyanide ATP synthase protons do not reduce O2, which acts as an electron acceptor |
ETS accepts energy from carriers in the matrix the ETS moves electrons because of the chemiosmotic gradient |
| ATP synthase | membrane-bound enzyme at final step in oxidative phosphorylation | follows from and is not part of the electron transport chain |
| ATP synthase activation | does not alter the chemiosmotic gradient | ETS maintains the gradient at a constant level ATP synthase activation increases the rate at which energy is removed from the gradient |
| O2 consumption | ATP is not needed for O2 consumption | O2 serves as an electron acceptor, so the electron transport chain drives O2 consumption |